Video Review
Key Concept Summary
TA Summary
Vocabulary
The structural organization of salts and metals.
The structural organization of Group 8A elements.
The structural organization of water.
The geometry around a carbon atom with four single bonds.
The geometry around a carbon atom with a triple bond.
The attractive interaction that holds atoms together in molecules.
True/False
Compounds containing transition metals are often brightly colored.
Sodium chloride (NaCl) and Magnesium (Mg) are examples of network substances.
Molecular substances melt at very high temperatures.
In a chemical formula, subscripts are placed to the left of the chemical symbol to indicate the number of that type of atoms in a molecule.
Mass spectrometry provides information about the energy associated with motions within molecules.
Analysis
Each molecule of nitric acid has the formula HNO3. If there are 6000 oxygen atoms present and 6000 nitrogen atoms present, how many hydrogen atoms will be required to convert all of the oxygen atoms into nitric acid?
For the pictures of molecules below, the gray balls represent carbon atoms and the white balls are hydrogen atoms. The molecules belong to a family called the hydrocarbons. These questions test if you can recognize patterns in molecular families and bonding around carbon atoms and then reason from them to make predictions.
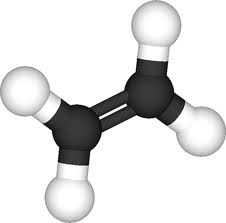
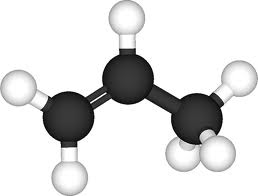
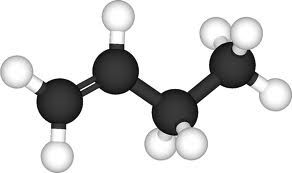
What is the formula for Ethene?
The chemical formula of the next family member after butylene will have what chemical formula?
Which of the following is NOT used as a technique for identifying chemical substances?
Which of the following is a mass spectrum for propylene?
Which of the following is atomic matter rather than molecular or network matter?
Which of the following is not a compound?
Two or more different elements that are chemically combined are always
The substance most likely to be a pure element is
Which of the following tends to have the highest melting and boiling points?
Which is the best definition of an alloy?