Video Review
Key Concept Summary
TA Summary
Vocabulary
Building block of most rocks.
Contains only C-C single bonds.
Silicate mineral that has a stringy structure.
Silicate mineral in which all silicate tetrahedra are bonded with equal strength to four other tetrahedra.
Silicate mineral that has a layer-like structure.
Best geometric arrangement of four bonds around a central atom.
State of matter in which atoms or ions are arranged in an orderly, repeating patterns.
Contain C=C double bonds.
True/False
Quartz is a silicate that has strong bonds throughout its entire structure.
Mica is a silicate that has strong bonds throughout its entire structure.
In minerals, the underlying atomic structure gives rise to large scale properties observable with the naked eye.
Quartz has fibrous layering in its atomic structure.
The melting point of saturated fatty acids increases with increasing number of carbon atoms.
An unsaturated fatty acid with the same number of carbon atoms as a saturated fatty acid will generally melt at a lower temperature than the saturated fatty acid.
Ionic bonds hold fatty acid molecules together in the solid state.
The -COOH group is what makes an organic acid an acid.
A silicate molecule is flat (planar)
The underlying atomic structure of both fatty acids and minerals determines many properties such as melting point and crystal morphology.
Most forms of asbestos form fibers.
Crystals of quartz flake off in thin sheets.
In the image below, the material has strong bonds where the pink balls occur and weak bonds where the blue balls occur. 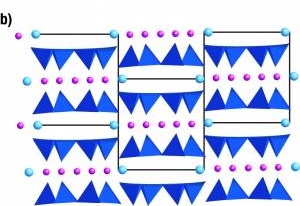

Fractures occur in crystals along lines or planes containing the strongest bonds.
The term "saturated" in saturated fats refers to whether every C atom has as many H atoms as it can hold.
Analysis
Which of the following would have the highest melting point?
Which of the following fatty acids is best for human health?
When you compare saturated with unsaturated fatty acids containing the same number of carbon atoms, what is true?
Which of the following structures would form a mineral such as mica that will flake off in sheets? 

The image below is of a silicate mineral. Make a prediction about whether this mineral would appear as fibers, layers, or no regular pattern. 

A triglyceride is made by combining
Which of the following is NOT a mineral?
Which of the following is a saturated fat?
Fatty acid molecules consist of a hydrocarbon tail attached to
Which of the following is NOT important in the determining the characteristics of fatty acids?