Video Review
Key Concept Summary
TA Summary
Vocabulary
Ions composed of more than one covalently-bound atom.
Forces between molecules.
A covalent bond involving two pairs of electrons shared between the two bound atoms.
Bonds or molecules having an unequal distribution of charge with one end being positive, the other negative.
The chemical bond between two non-metals characterized by sharing of valence electrons.
Chemical compounds made up primarily of the elements carbon and hydrogen.
A linear system that has a positive end and a negative end.
A material in which another material dissolves.
Having a pH value less than 7, meaning that the hydronium ion concentration is greater than in pure water.
True/False
Metal and non-metal substances combine to form covalent compounds
Covalent molecules do not have well-defined shapes.
The triple covalent bond of carbon monoxide (CO) is a type of weak chemical interaction.
Molecules are only formed by covalent bonds.
Dispersion forces are stronger than covalent bonds.
N2 has a triple bond and is a gas at room temperature. True or False: The triple bond is a very strong bond.
The forces between the N2 molecules in a container of N2 are very strong.
Analysis
When some Ca(NO3)2 dissolves in water which picture best represents what the solution looks like? (Note, the balls are not meant to indicate charge or lack of charge.) 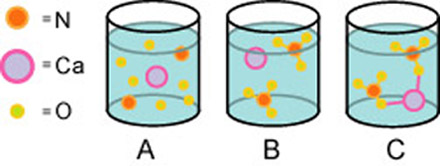

Which of the following is a true statement about melting and boiling points in materials made of covalent molecules?
If carbon is combined with oxygen, it forms a new molecular material. In the next six questions, you are asked to make some predictions about the characteristics and behavior of this molecule.
What type of bond would exist between the carbon and the oxygen?
Suppose the carbon and oxygen react according to the equation: 6O2 + C6 = 6CO2. If you started with sixty oxygen molecules (60O2) and sixty graphene(60C6), how many carbon dioxide molecules could you make?
Which of the following would best describe the characteristics of the material formed?
Electronegativities are shown in the chart below. What can you conclude about the molecule? 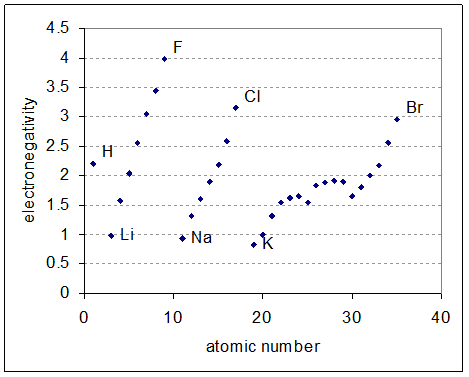

What best describes how the carbon dioxide molecules would behave if mixed with water?
Which of the following images correctly depicts the reaction 6O2 + C6 = 6CO2?
Which type of bonding involves the sharing of electrons?
Which of these substances consists of individual molecules?
Which of the following pairs would you expect to be covalently bonded?
Which of the following set of properties is most likely those associated with methane, a covalently bonded compound?
Rank the following according to their strength, weakest first, strongest last.