Video Review
Key Concept Summary
TA Summary
Vocabulary
A uranium atom splits into thorium and helium.
A uranium atom splits into xenon and strontium plus a few neutrons.
A process where one event always triggers one or more additional events.
The minimum amount of mass necessary for one fission to trigger another.
A neutron changes into a proton and an electron.
Two hydrogen atoms combine to make a helium atom.
A nucleon jumps from a high energy level to a lower one and emits a photon.
The amount of time required for 1/2 of the remaining radioactive nuclei to decay.
True/False
The strong force between two protons is stronger than the electric repulsion between two protons.
The electromagnetic force extends over a longer distance than the strong nuclear force.
Charge is always conserved in an nuclear process.
Mass changes in nuclear processes.
If two large nuclei were to combine, the process would release energy.
It is possible to predict exactly when a radioactive nucleus will decay.
Analysis
Which of the following is a legitimate radioactive decay because both charge and mass number are conserved?
Why is a high temperature required for nuclear fusion to occur?
The purpose of the control rods in a nuclear fission reactor is
Uranium-238 alpha decays, emitting a helium-4 nucleus. What is the mass number of the product?
The process known as nuclear fission is primarily responsible for
Both fission and fusion can result in a release of energy. Why?
When you compare the mass of the products to the mass of the reactants in a fission reaction, what do you find? (note the question is asking about mass, not mass number)
Carbon-14 (14C) is not stable and it spontaneously decays into nitrogen-14 (14N) and an electron by a radioactive beta-decay. Which of the following is true about the decay?
From the graphical information presented below, estimate the half-life of the radioactive element whose decay curve is plotted. 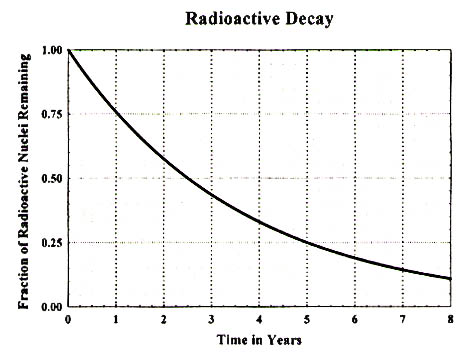

In which of the following nuclear reactions is total charge conserved?
Carbon-14 is a radioactive isotope of carbon. Nitrogen-14 is naturally occurring. If you compare the two, what would you expect to find?
Which of the following nuclear reactions has a proton changed into a neutron?
Which of the following is true regarding forces in nuclei?
Which of the following would be the best fuel for a fission reaction?
Which of the following is the biggest technical problem with using fusion as a source of energy?
An unstable nucleus which has a tendency to spontaneously change its form with the emission of high-energy particles or photons is said to be
The process that produces most of the explosive energy of a hydrogen bomb, and that is believed to be responsible for the energy released by the sun, is called
Isotopes of an element are different atoms of the element that
Nuclear fission is when
What is the approximate range of the nuclear strong force? (In other words, what is the distance over which the strong force is significant?)